Ensuring Quality of ISO 15189 Examination Results
Medical laboratories are essential for proper patient care and accurate results must be obtained in order to to take the right course of action to treat any patient. To ensure that medical laboratories are ready to meet all the needs that patients might have, medical laboratories must pass ISO 15189 Examinations.
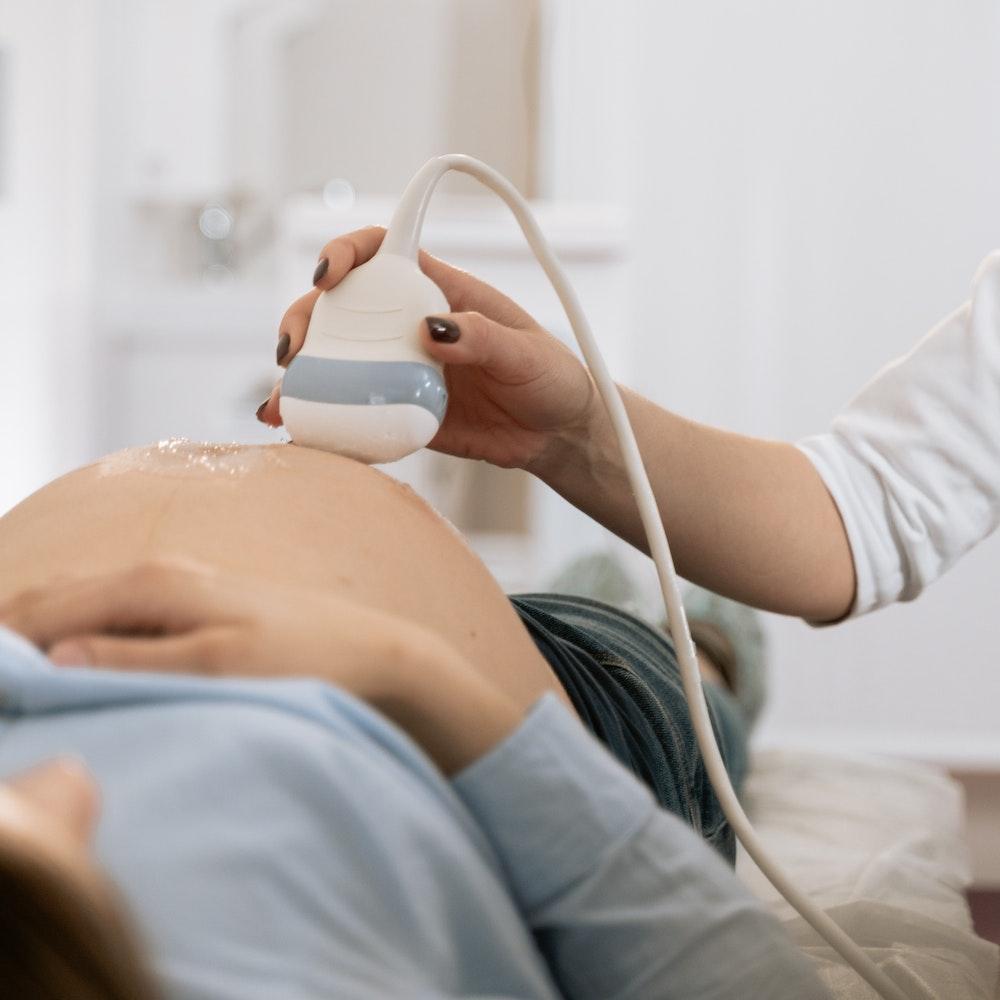
Quality control materials, along with patient samples, should be examined at appropriate intervals based on the procedure’s stability. The laboratory should select control material concentrations, particularly those at or near clinical decision values, to ensure the validity of decisions made. The use of independent third-party control materials, either instead of or in addition to any control materials supplied by the reagent or instrument manufacturer, should be considered. The basic idea behind the control sample system is that quality control materials are processed in the same way that patient samples are. The quality control materials’ results should not exceed tolerance limits (permissible deviations of measurements).
The laboratory management is in charge of defining the tolerance limits. These limits, as well as the reasoning behind their establishment, should be documented. When quality control rules are broken, examination results should be rejected, and relevant patient samples should be re-examined after the error condition has been corrected and within-specification performance has been verified. The laboratory should also evaluate the results of patient samples examined following the most recent successful quality control event.
Quality control data should be reviewed and monitored on a regular basis in order to detect trends in examination performance that could indicate problems with the examination system. When such trends are identified, preventive measures should be implemented and documented. Wherever possible, established statistical techniques and process control rules should be used to continuously monitor examination system performance. Qualitative tests necessitate specific internal quality control procedures.
Qualitative examinations are those that assess cellular characteristics such as morphology or measure the presence or absence of a substance. The outcomes are not expressed numerically, but rather descriptively or qualitatively as positive, negative, reactive, nonreactive, normal, or abnormal. Many of these tests do not lend themselves as easily to quality control as quantitative tests do. As a result, in addition to traditional quality control methods, it is critical to carefully monitor other processes within the quality system.
The following are some key quality concepts that apply to both qualitative and semi-quantitative tests in ISO 15189 Examination.:
- In all laboratory testing, sample management is critical.
- Examinations requiring a viable organism in the sample may necessitate close monitoring and effective communication with non-laboratory personnel/departments.
- Incubators, refrigerators, microscopes, autoclaves, and other equipment must be kept in good working order and closely monitored.
- To monitor the effectiveness of test procedures that use special stains or reagents, as well as tests with endpoints such as agglutination, colour change, or other non-numerical results, positive and negative controls must be used.
- Reagents should be stored according to manufacturer’s instructions, labelled with the date opened and used, and discarded when they reach their expiration date.
Qualitative examinations necessitate a wide range of control materials. Built-in (onboard or procedural) controls, traditional controls that mimic patient samples, or stock cultures for use with microbiological examinations are examples of these.
External Quality Assessment Schemes in ISO 15189 Examination
The laboratory should take part in external quality assurance (EQA) programmes (or proficiency testing programmes) that are relevant to the examinations and interpretations offered. The laboratory should develop a documented procedure outlining the responsibilities and instructions for participating in external quality assurance schemes. The basic idea behind the control sample system is that quality control materials are processed in the same way that patient samples are.
External quality assessment schemes should be run on both primary and backup analyzers, if available. When control criteria are not met, the laboratory director should monitor the results of the external quality assessment schemes and participate in the implementation of corrective actions. Laboratories should formalize which actions are taken and who is responsible for the outcomes by signing them (e.g., responsible technicians, quality manager, and laboratory or unit head).
Laboratories should share the results of external quality assessment reports with all affected personnel to inform them of gaps and corrective actions taken. It is recommended that samples obtained from external quality assessment scheme providers be measured at two different levels for each parameter at least four times per year. Laboratories that do not check bias in each cycle within their internal controls should participate in external quality assessment schemes six times per year.